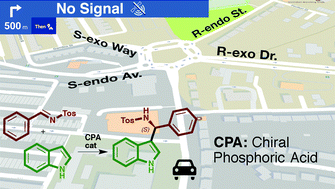
Enantioselectivity in CPA-catalyzed Friedel–Crafts reaction of indole and N-tosylimines: a challenge for guiding models†
Abstract
Qualitative reaction models or predicting guides are a very useful outcome of theoretical investigations of organocatalytic reaction mechanism that allow forecasting of the degree and sense of the enantioselectivity of reactions involving novel substrates. However, application of these models can be unexpectedly challenging in reactions affected by a large number of conformations and potential control of the enantioselectivity by different reaction steps. The QM/MM study of the Friedel–Crafts reaction between indole and the N-tosylimide of benzaldehyde catalysed by different CPA reveals that the reaction consists of two CPA-assisted steps: the addition of the two reagents to yield a Wheland intermediate, and its re-aromatization. The relevance of the second step depends on the catalyst: it changes the sense of the expected stereoselectivity for a BINOP-derived CPA but is irrelevant in the reaction catalysed by a VAPOL-derived imidodiphosphoric acid catalyst. Although the relative energies of the TSs can be rationalized considering the steric interactions with the catalyst, the possibility of additional H-bonds, or the relative stability of the conformation of the reagents, predicting the enantioselectivity is not possible using qualitative guides.

 Please wait while we load your content...
Please wait while we load your content...