Conformational stabilization of a β-hairpin through a triazole–tryptophan interaction†
Abstract
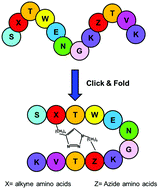
Molecular tools to stabilize the β-hairpin conformation are needed as β-hairpin peptides are useful molecules for pharmaceutical, biological and materials applications. We explored the use of a “triazole bridge”, a covalent link between two β-hairpin strands obtained through Cu-catalyzed alkyne–azide cycloaddition, combined with an aromatic–aromatic interaction. Highly conformationally stable peptides were identified by NMR screening of a small collection of cyclic peptides based on the Trpzip2 scaffold. The characteristic Trp–Trp interaction of Trpzip2 was replaced by a diagonal triazole bridge of variable length. NMR and CD analyses showed that triazole and indole rings could favorably interact to stabilize a β-hairpin conformation. The conformational stabilization depends on the length of the triazole bridge and the reciprocal position between the aromatic rings. Combining aromatic interactions and the covalent inter-strand triazole bridge is a useful strategy to obtain peptides with a high β-hairpin content.


 Please wait while we load your content...
Please wait while we load your content...