Design and synthesis of a hybrid framework of indanone and chromane: total synthesis of a homoisoflavanoid, brazilane†
Abstract
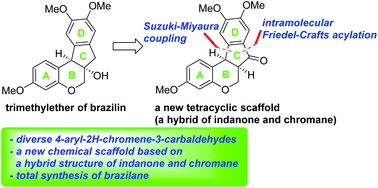
A chemical backbone of tetracyclic homoisoflavanoid natural products such as brazilin inspired us to design a new chemical scaffold, 6a,11b-dihydroindeno[2,1-c]chromen-7(6H)-one, which is a hybrid structure of indanone and chromane. Pd-catalyzed Suzuki–Miyaura cross-coupling of 4-chloro-2H-chromene-3-carbaldehydes with (hetero)aryl boronic acids was employed as a means to introduce a wide variety of (hetero)aryl groups as the D ring and intramolecular Friedel–Crafts acylation was utilized to construct the C ring of this skeleton. Total synthesis of the natural product, brazilane, was also demonstrated via this new chemical framework.


 Please wait while we load your content...
Please wait while we load your content...