Hydroxyl directed C-arylation: synthesis of 3-hydroxyflavones and 2-phenyl-3-hydroxy pyran-4-ones under transition-metal free conditions†
Abstract
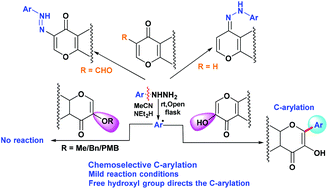
An efficient, transition-metal free and direct C-arylation of 3-hydroxychromone moieties in the presence of a base, air as an oxidant and arylhydrazines as arylating agents to furnish highly biologically active flavonols or 3-hydroxyflavones has been developed. We have further extended our methodology for the C-arylation of the 5-hydroxy pyran-4-one moiety. The role of the free hydroxyl group towards C-arylation has been delineated.


 Please wait while we load your content...
Please wait while we load your content...