Metal-free oxidative para-acylation of unprotected anilines with N-heteroarylmethanes†
Abstract
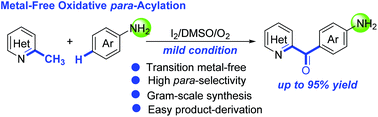
A selective oxidative para-acylation of unprotected anilines with methyl groups in N-heteroarylmethanes was achieved. This transformation proceeds under mild metal-free reaction conditions to produce the corresponding valuable diarylmethanones in good to high yields, featuring high site-selectivity, high functional-group-tolerance, gram-scale synthesis and easy product-derivation. Preliminary mechanistic studies revealed that the present oxidative para-acylation would take place via a Friedel–Crafts-type process of in situ imines and the steric hindrance might be the key issue for the high regio-selectivity.


 Please wait while we load your content...
Please wait while we load your content...