A novel sulfonamide non-classical carbenoid: a mechanistic study for the synthesis of enediynes†
Abstract
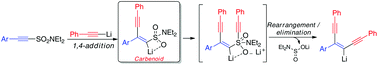
Alkynyl sulfonamides undergo sequential 1,4- then 1,2-addition/rearrangement with lithium acetylides to yield enediynes in the absence of any promoters or catalysts. Mechanistic investigations suggest that the reaction proceeds via 1,4-conjugate addition of the nucleophile to the unsaturated system to give a key alkenyl lithium species which is stabilised by an intramolecular coordination effect by a sulfonamide oxygen atom. This species can be considered a vinylidene carbenoid given the carbon atom bears both an anion (as a vinyllithium) and a leaving group (the sulfonamide). The intramolecular coordination effect serves to stabilise the vinyllithium but activates the sulfonamide motif towards nucleophilic attack by a second mole of acetylide. The resulting species can then undergo rearrangement to yield the enediyne framework in a single operation with concomitant loss of aminosulfinate.


 Please wait while we load your content...
Please wait while we load your content...