Regioselective three-component synthesis of 2,3-disubstituted quinolines via the enaminone modified Povarov reaction†
Abstract
The regioselective construction of functionalized quinolines by the three-component reactions of enaminones, aldehydes and anilines is accomplished. Unlike conventional Povarov reactions employing terminal alkynes or alkenes as C3–C4 fragment sources which provide 2,4-disubstituted quinolines, the present method allows fast and regioselective formation of 2,3-disubstituted quinolines as a modified new version of the Povarov reaction.
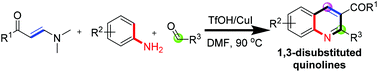


 Please wait while we load your content...
Please wait while we load your content...