Peramivir analogues bearing hydrophilic side chains exhibit higher activities against H275Y mutant than wild-type influenza virus†
Abstract
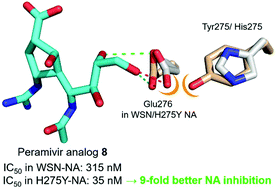
Peramivir is an effective anti-influenza drug in the clinical treatment of influenza, but its efficacy toward the H275Y mutant is reduced. The previously reported cocrystal structures of inhibitors in the mutant neuraminidase (NA) suggest that the hydrophobic side chain should be at the origin of reduced binding affinity. In contrast, zanamivir having a hydrophilic glycerol side chain still possesses high affinity toward the H275Y NA. We thus designed five peramivir analogues (5–9) carrying hydrophilic glycol or glycerol side chains, and evaluated their roles in anti-influenza activity, especially for the H275Y mutant. The synthetic sequence involves a key step of (3 + 2) cycloaddition reactions between alkenes and nitrile oxides to construct the scaffold of peramivir carrying the desired hydrophilic side chains and other appropriate functional groups. The molecular docking experiments reveal that the hydrophilic side chain can provide extra hydrogen bonding with the translocated Glu-276 residue in the H275Y NA active site. Thus, the H275Y mutant may be even more sensitive than wild-type virus toward the peramivir analogues bearing hydrophilic side chains. Notably, the peramivir analogue bearing a glycerol side chain inhibits the H275Y mutant with an IC50 value of 35 nM, which is better than the WSN virus by 9 fold.


 Please wait while we load your content...
Please wait while we load your content...