Dissociative reactions of benzonorbornadienes with tetrazines: scope of leaving groups and mechanistic insights†
Abstract
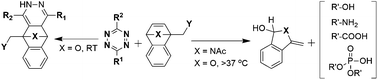
Bioorthogonal dissociative reactions boast diverse potential applications in chemical biology and drug delivery. The reaction of benzonorbornadienes with tetrazines to release amines from carbamate leaving groups was recently introduced as a bioorthogonal bond-cleavage reaction. The present study aimed at investigating the scope of leaving groups that are compatible with benzonorbornadienes. Synthesis of several benzonorbornadienes with different releasable groups is reported, and the reaction of these molecules with tetrazine was found to be rapid and afforded high release yields. The tetrazine-induced release of molecules proceeds in a cascade of steps including inverse-electron demand cycloaddition and cycloreversion reactions that form unstable isoindoles/isobenzofuran intermediates and spontaneously eliminate a leaving group of interest. In the case of oxygen-bridged BNBDs at room temperature, we observed the formation of an unproductive byproduct.


 Please wait while we load your content...
Please wait while we load your content...