Multicomponent, fragment-based synthesis of polyphenol-containing peptidomimetics and their inhibiting activity on beta-amyloid oligomerization†
Abstract
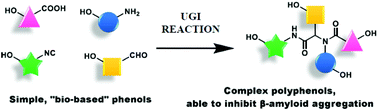
A new and short fragment-based approach towards artificial (but “natural-based”) complex polyphenols has been developed, exploiting the Ugi multicomponent reaction of phenol-containing simple substrates. The resulting library of compounds has been tested for its capacity to inhibit β-amyloid protein aggregation, as a possible strategy to develop new chemical entities to be used as prevention or therapy for Alzheimer's disease. Some of the members of this library have demonstrated, in thioflavin assays, a highly promising activity in inhibiting aggregation for two β-amyloid peptides: Aβ1-42 and the truncated AβpE3-42.


 Please wait while we load your content...
Please wait while we load your content...