Cu-Catalyzed asymmetric Friedel–Crafts propargylic alkylation of phenol derivatives†
Abstract
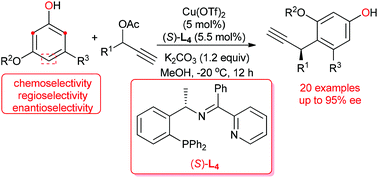
A copper-catalyzed asymmetric Friedel–Crafts propargylic alkylation of electron-rich phenol derivatives with a variety of propargylic esters has been described. With Cu(OTf)2 decorated with a chiral tridentate ketimine P,N,N-ligand as the catalyst, asymmetric Friedel–Crafts propargylic alkylation of 3,5-dialkoxyphenol derivatives proceeded smoothly in high yields and with good to excellent enantioselectivities. The present study suggested that the presence of an electron-rich substituent on the meta-position of phenol is essential for the promotion of Friedel–Crafts propargylic alkylation, and the substrate bearing two electron-rich groups on both the 3,5-positions of phenol tends to give a satisfactory performance.


 Please wait while we load your content...
Please wait while we load your content...