Synthetic approaches to the C11–C27 fragments of bryostatins†
Abstract
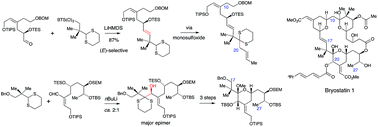
The modified Julia reaction and acyl carbanion chemistry, especially reactions of 2-lithiated dithianes, have been investigated for the synthesis of intermediates that are the synthetic equivalents of the C11–C27 fragments of bryostatins. The modified Julia reaction using 2-benzothiazolylsulfones was found to be more useful for the formation of the C16–C17 double-bond than the classical Julia reaction using phenylsulfones, and bulky sulfones gave very good (E)-stereoselectivity. The alkylation of a dithiane monoxide that corresponded to a C19-acyl carbanion using (E)-1-bromobut-2-ene was efficient but the use of a more complex allylic bromide corresponding to the C20–C27 fragment of the bryostatins was unsuccessful, possibly due to competing elimination reactions. This meant that the use of C19 dithianes for the synthesis of 20-deoxybryostatins would have to involve the stepwise assembly of the C20–C27 fragment from simpler precursors. However, lithiated C19 dithianes gave good yields of adducts with aldehydes and conditions were developed for the stereoselective conversion of the major adducts into methoxyacetals that corresponded to the C17–C27 fragment of 20-oxygenated bryostatins. A convergent synthesis of the C11–C27 fragment of a 20-deoxybryostatin was subsequently achieved using a 2-benzothiazolylsulfone corresponding to the intact C17–C27 fragment.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...