Tuning the tautomeric behavior of tris(salicylaldimines)†
Abstract
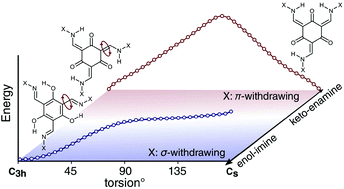
Five new tris(N-salicylaldimine) (TSAN) analogues were prepared and characterized. NMR and single-crystal X-ray diffraction studies showed that they are found in different tautomeric forms, ranging from keto–enamine to enol–imine, with two showing intermediate behavior. We present a simple structural model governing the relative stability of the keto–enamine versus enol–imine tautomeric form of TSANs, based on experimental and theoretical findings on the new and existing TSAN analogues. Examination of electron delocalization throughout this range reveals a connection between tautomeric state and whether the substituent is σ or π electron withdrawing/donating. This can be used as a qualitative guide to design TSANs with controlled tautomeric behavior. These results will be helpful to the growing number of researchers in supramolecular chemistry who use TSANs to construct new materials and cages.


 Please wait while we load your content...
Please wait while we load your content...