N-Heterocyclic carbene palladium-catalyzed cascade annulation/alkynylation of 2-alkynylanilines with terminal alkynes†
Abstract
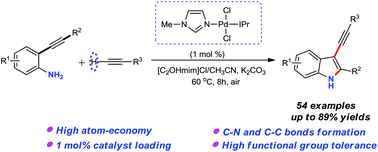
A straightforward and highly effective N-heterocyclic carbene-palladium catalyzed cascade annulation/alkynylation of 2-alkynylanilines with terminal alkynes has been enabled to afford free (NH)-3-alkynylindole derivatives in moderate to good yields. This protocol features mild conditions, broad substrate scope, and high atom- and step-economy. Notably, the resultant 3-alkynylindoles could be conveniently transformed into a variety of functionalized indole scaffolds, thus illustrating their potential applications in synthetic and pharmaceutical chemistry.


 Please wait while we load your content...
Please wait while we load your content...