Synthesis of quinolines via copper-catalyzed domino reactions of enaminones†
Abstract
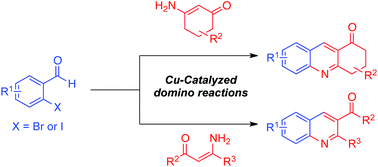
Quinoline derivatives were obtained from enaminones and 2-bromo- or 2-iodobenzaldehydes via copper-catalyzed domino reactions consisting of the aldol reaction, C(aryl)–N bond formation and elimination. The electronic effect of aldehydes played a major role in the reaction outcome. Two simple protocols are disclosed to achieve various quinolines from both cyclic and acyclic enaminones in good yields. With the less-reactive acyclic enaminones, diethyl-2-(2-bromobenzylidene)malonate was shown to be more compatible than the benzaldehydes.


 Please wait while we load your content...
Please wait while we load your content...