Enantioselective bioreduction of benzo-fused cyclic ketones with engineered Candida glabrata ketoreductase 1 – a promising synthetic route to ladostigil (TV3326)†
Abstract
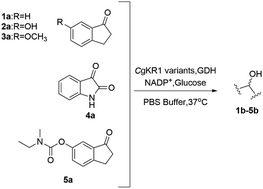
Biocatalysis has been recently emerging as a promising alternative to traditional chemical synthesis because of its “green” characteristics and comparable selectivities, which accord with the concept of sustainable development and demand for asymmetric synthesis. In this study, whole-cell biocatalysts containing glucose dehydrogenase (GDH) and Candida glabrata ketoreductase 1 (CgKR1) variants were constructed. These biocatalysts were applied to the reduction of benzo-fused cyclic ketones and showed good to high activities and enantioselectivities. Particularly, CgKR1 variants displayed high activities (90.6%–98.4% conversions) and enantioselectivities (>99.9% ee) towards 5a, a key intermediate of ladostigil (TV3326). Based on these results, a chemoenzymatic synthesis of (S)-5b was developed by using biocatalytic asymmetric reduction as a key step, giving the product with a total yield of 34.0% and 99.9% ee.


 Please wait while we load your content...
Please wait while we load your content...