Visible light catalyzed Mannich reaction between tert-amines and silyl diazoenolates†
Abstract
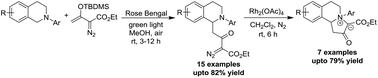
The present work documents the α-C–H functionalization of tertiary amines via the visible light catalyzed Mannich reaction with silyl diazoenolates. The reaction takes place at room temperature with an organic dye, Rose Bengal, as a photocatalyst and oxygen as the oxidant. The resulting multifunctional products bearing an α-diazo-β-keto group undergo Rh-carbenoid mediated cyclization, affording stable ammonium ylides in high yields.


 Please wait while we load your content...
Please wait while we load your content...