Simulating the reactions of substituted pyridinio-N-phosphonates with pyridine as a model for biological phosphoryl transfer†
Abstract
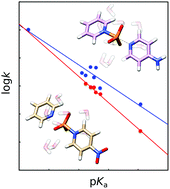
Phosphoryl transfer reactions can proceed through several plausible mechanisms, and the potential for both solvent and substrate-assisted pathways (involving proton transfer to the phosphoryl oxygens) complicates both experimental and computational interpretations. To avoid this problem, we have used electronic structure calculations to probe the mechanisms of the reactions of pyridinio-N-phosphonates with pyridine. These compounds avoid the additional complexity introduced by proton transfer between the nucleophile and the leaving group, while also serving as a valuable model for biological P–N cleavage. Through a comparative study of a range of substrates of varying basicity, we demonstrate a unified concerted mechanism for the phosphoryl transfer reactions of these model compounds, proceeding through a dissociative transition state. Finally, a comparison of these transition states with previously characterized transition states for related compounds provides a more complete model for non-enzymatic phosphoryl transfer, which is a critical stepping stone to being able to fully understand phosphoryl transfer in biology.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...