Asymmetric synthesis of α-deuterated α-amino acids†
Abstract
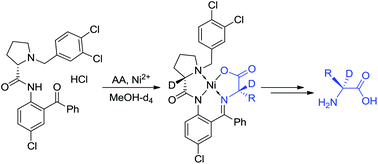
α-Deuterated-α-amino acids represent a very special class of stable isotopically labeled compounds, used in advanced biomedical research. Herein, we disclose a generalized approach for the preparation of α-2H-α-amino acids in enantiomerically pure form and with up to 99% deuteration. The reaction chemistry involved in this process is based on the dynamic kinetic resolution of racemates or (S)–(R) interconversion via the formation of intermediate Ni(II) complexes derived from unprotected amino acids and recyclable tridentate ligands. Operationally convenient conditions, excellent chemical yields, diastereoselectivity and the degree of the deuteration bode well for the wide application of this methodology for the preparation of tailor-made α-2H-α-amino acids.


 Please wait while we load your content...
Please wait while we load your content...