N- to C-sulfonyl photoisomerisation of dihydropyridinones: a synthetic and mechanistic study†
Abstract
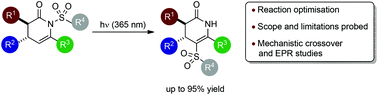
The scope and limitations of a photoinitiated N- to C-sulfonyl migration process within a range of dihydropyridinones is assessed. This sulfonyl transfer proceeds without erosion of either diastereo- or enantiocontrol, and is general across a range of N-sulfonyl substituents (SO2R; R = Ph, 4-MeC6H4, 4-MeOC6H4, 4-NO2C6H4, Me, Et) as well as C(3)-(aryl, heteroaryl, alkyl and alkenyl) and C(4)-(aryl and ester) substitution. Crossover reactions indicate an intermolecular step is operative within the formal migration process, although no crossover from C-sulfonyl products was observed. EPR studies indicate the intermediacy of a sulfonyl radical and a mechanism is proposed based upon these observations.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...