Solvent controlled radical cyclization of propargylamines for multi-iodinated quinoline formation†
Abstract
A solvent controlled regioselective metal-free synthesis of iodo-substituted N-heterocycles has been developed. This protocol undergoes a cascade iodination/cyclization/oxidation/aromatization pathway to afford multi-halogenated quinolines from readily available propargylamines under mild conditions.
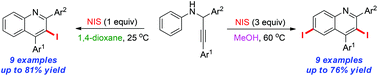


 Please wait while we load your content...
Please wait while we load your content...