Copper-catalyzed transformation of ketones to amides via C(CO)–C(alkyl) bond cleavage directed by picolinamide†
Abstract
Copper catalyzed chemoselective cleavage of the C(CO)–C(alkyl) bond leading to C–N bond formation with chelation assistance of N-containing directing groups is described. Inexpensive Cu(II)-acetate serves as a convenient catalyst for this transformation. This method highlights the emerging strategy to transform unactivated alkyl ketones into amides in organic synthesis and provides a new strategy for C–C bond cleavage.
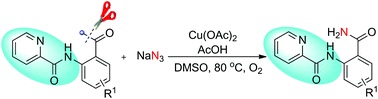


 Please wait while we load your content...
Please wait while we load your content...