Copper-catalyzed synthesis of arylcarboxamides from aldehydes and isocyanides: the isocyano group as an N1 synthon†
Abstract
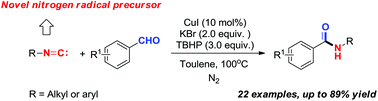
An interesting radical coupling reaction of aromatic aldehydes with isocyanides was disclosed for the synthesis of amides catalyzed by copper. According to the experimental results and mechanistic study, the isocyano group acted as an N1 synthon rather than exhibiting the carbene-like reactivity, exploiting a new reactivity profile of isocyanides.


 Please wait while we load your content...
Please wait while we load your content...