First stereoselective total synthesis of brevipolide M†
Abstract
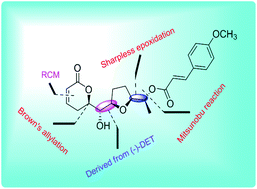
The first stereoselective total synthesis of a cytotoxic brevipolide M, which shares a pyrone framework bearing a tetrahydrofuran moiety and a cinnamate group with the readily available (−)-DET, is described. The key steps involved in the synthesis are the epoxide-opening, Brown's allylation, and the RCM reaction to install an α,β-unsaturated lactone ring and the inversion of the C-6′ stereogenic hydroxyl group using the Mitsunobu reaction.


 Please wait while we load your content...
Please wait while we load your content...