Structural and mechanistic studies of the base-induced Sommelet–Hauser rearrangement of N-α-branched benzylic azetidine-2-carboxylic acid-derived ammonium salts†
Abstract
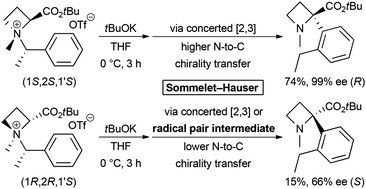
The base-induced Sommelet–Hauser rearrangement of N-α-branched benzylic azetidine-2-carboxylic acid ester-derived ammonium salts to obtain α-arylazetidine-2-carboxylic acid esters was investigated. The substrates, two diastereomeric salts (1S,2S,1′S)- and (1R,2R,1′S)-2, showed different reactivities. The rearrangement of (1S,2S,1′S)-2a proceeded with a perfect N-to-C chirality transfer to provide (R)-3a in 74% yield with 99% ee. However, the rearrangement of (1R,2R,1′S)-2a under the same conditions afforded (S)-3a in only 15% yield with a lower 66% ee, along with the competitive [1,2] Stevens rearrangement product 4a. Structural and mechanistic studies of this rearrangement were carried out to clarify the exact reason. Our results define the scope and limitations of the Sommelet–Hauser rearrangement and provide unique synthetic access to α-aryl amino acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...