N-Hydroxy sulfonamides as new sulfenylating agents for the functionalization of aromatic compounds†
Abstract
An unprecedented use of N-hydroxy sulfonamides as sulfenylating agents has been established. In the presence of catalytic amounts of iodine and N-hydroxysuccinimide, N-hydroxy sulfonamides participated in sulfenylation with indoles, 7-azaindole, N-methyl pyrrole, and 2-naphthol to afford structurally diverse thioethers in moderate to excellent yields with very high regioselectivity.
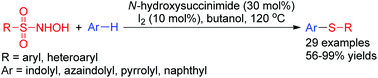


 Please wait while we load your content...
Please wait while we load your content...