Excited-state intermolecular proton transfer dependent on the substitution pattern of anthracene–diurea compounds involved in fluorescent ON1–OFF–ON2 response by the addition of acetate ions†
Abstract
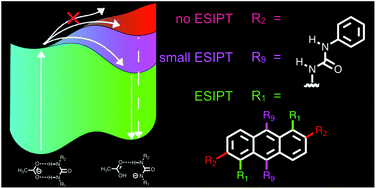
We report anthracene–diurea compounds which behave as anion sensors based on the fluorescence emission regulated by the substitution position on the anthracene ring. Anthracene–diurea compounds exhibit different excited-state intermolecular proton transfer (ESIPT) reactions depending on the pattern of the substituents. Three new anthracene–diurea compounds that have two phenylurea groups substituted at different positions on anthracene were synthesized. These compounds formed complexes with acetate ions through intermolecular hydrogen bonding between N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moieties in the ground state. The positions of the substituents greatly affected the excited-state intermolecular proton transfer. 1,5BPUA with urea groups at the 1 and 5 positions exhibited ESIPT reaction, which is energetically favorable for tautomer formation, in the presence of TBAAc. In contrast, 2,6BPUA with urea groups at low-electron-density positions (2 and 6 positions) showed no ESIPT reaction due to the inversion of the lowest unoccupied molecular orbital (LUMO) energy levels of the normal and tautomer states. Detailed spectroscopic measurements showed that the LUMO energy level of the normal form was lowered because the urea group acted as an electron-withdrawing group. In addition, 9,10BPUA exhibited strong electronic interactions between the two phenylurea moieties at the 9 and 10 positions, resulting in an ON1–OFF–ON2 response for acetate ions. Our findings offer guidelines for the molecular design of materials with anthracene moieties based on the substitution patterns of anthracene derivatives.
O moieties in the ground state. The positions of the substituents greatly affected the excited-state intermolecular proton transfer. 1,5BPUA with urea groups at the 1 and 5 positions exhibited ESIPT reaction, which is energetically favorable for tautomer formation, in the presence of TBAAc. In contrast, 2,6BPUA with urea groups at low-electron-density positions (2 and 6 positions) showed no ESIPT reaction due to the inversion of the lowest unoccupied molecular orbital (LUMO) energy levels of the normal and tautomer states. Detailed spectroscopic measurements showed that the LUMO energy level of the normal form was lowered because the urea group acted as an electron-withdrawing group. In addition, 9,10BPUA exhibited strong electronic interactions between the two phenylurea moieties at the 9 and 10 positions, resulting in an ON1–OFF–ON2 response for acetate ions. Our findings offer guidelines for the molecular design of materials with anthracene moieties based on the substitution patterns of anthracene derivatives.


 Please wait while we load your content...
Please wait while we load your content...