Ru-Catalyzed highly diastereoselective hydrogenation of N-tert-butylsulfinyl ketimines for the synthesis of aryl glycine derivatives†
Abstract
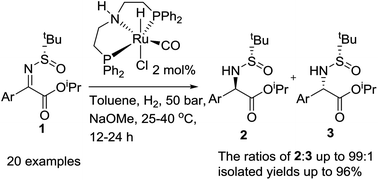
A ruthenium pincer catalyst has been shown to be highly efficient for the hydrogenation of a wide range of α-ketimino esters derived from α-keto esters and chiral 2-methylpropyl-2-sulfinamide, affording chiral aryl glycine derivatives with high yields and diasteroselectivities (20 examples, dr values up to 99 : 1).


 Please wait while we load your content...
Please wait while we load your content...