Dissociation of Mg(ii) and Zn(ii) complexes of simple 2-oxocarboxylates – relationship to CO2 fixation, and the Grignard and Barbier reactions†
Abstract
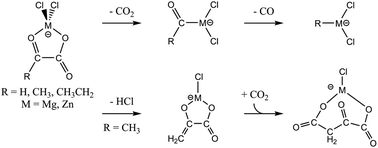
Three deprotonated 2-oxocarboxylic acids, glyoxylate, pyruvate, and 2-oxobutyrate (RCOCO2−, R = H, CH3, CH3CH2) have been associated with MgCl2 and ZnCl2 to generate [RCOCO2MCl2]− (M = Mg, Zn) complexes. Upon collision-induced dissociation these complexes all undergo efficient eliminations of CO2 and CO, via an intermediate [RCOMCl2]− product, to ultimately give [RMCl2]− products. The pyruvate and 2-oxobutyrate complexes also undergo efficient elimination of HCl to produce the enolate-metal complexes [H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) COCO2MCl]− and [H3CHC
COCO2MCl]− and [H3CHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) COCO2MCl]−. These enolate complexes have binding motifs reminiscent of the active centres in some CO2-fixating enzymes and the CO2 reactivity of these enolate complexes was therefore investigated, but only adduct formation could be observed. Quantum chemical calculations predict the magnesium complexes to decarboxylate without reverse barriers to carboxylation, and the zinc complexes to decarboxylate with considerable reverse barriers. The subsequent CO loss occurs with reverse barriers in all cases. The HCl loss is also predicted to occur overall without reverse barriers for both metals.
COCO2MCl]−. These enolate complexes have binding motifs reminiscent of the active centres in some CO2-fixating enzymes and the CO2 reactivity of these enolate complexes was therefore investigated, but only adduct formation could be observed. Quantum chemical calculations predict the magnesium complexes to decarboxylate without reverse barriers to carboxylation, and the zinc complexes to decarboxylate with considerable reverse barriers. The subsequent CO loss occurs with reverse barriers in all cases. The HCl loss is also predicted to occur overall without reverse barriers for both metals.


 Please wait while we load your content...
Please wait while we load your content...