Chiral squaramide-catalysed enantioselective Michael/cyclization cascade reaction of 3-hydroxyoxindoles with α,β-unsaturated N-acylated succinimides†
Abstract
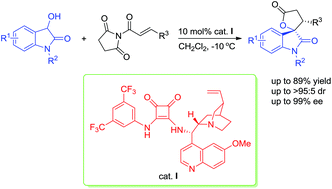
A bifunctional squaramide-catalysed asymmetric Michael/cyclization cascade reaction of 3-hydroxyoxindoles with α,β-unsaturated N-acylated succinimides is disclosed. With quinine-derived squaramide as the catalyst, a broad range of the desired spirooxindole lactone derivatives bearing two contiguous stereocenters were obtained in good yields (up to 89%) with high diastereoselectivities (up to >95 : 5 dr) and excellent enantioselectivities (up to 99% ee).


 Please wait while we load your content...
Please wait while we load your content...