Exploring the catalytic mechanism of dihydropteroate synthase: elucidating the differences between the substrate and inhibitor†
Abstract
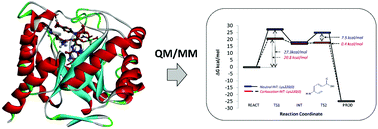
Dihydropteroate synthase (DHPS) catalyzes the condensation of 6-hydroxymethyl-7,8-dihydropterin pyrophosphate (DHPPP) with p-aminobenzoic acid (pABA) and is a well validated target for anti-malarial and anti-bacterial drugs. However, in recent years its utility as a therapeutic target has diminished considerably due to multiple mutations. As such, considerable structural biology and medicinal chemistry effort has been expended to understand and overcome this issue. To date no detailed computational analysis of the protein mechanism has been made despite the detailed crystal structures and multiple mechanistic proposals being made. In this study the mechanistic proposals for DHPS have been systematically investigated using a hybrid QM/MM method. We aimed to compare the energetics associated with SN1 and SN2 processes, whether the SN1 process involves a carbocation or neutral DHP intermediate, uncover the identity of the general base in the catalytic mechanism, and understand the differences in substrate vs. inhibitor reactivity. Our results suggest a reaction that follows an SN1 process with the rate determining step being C–O bond breaking to give a carbocation intermediate. Comparative studies on the inhibitor STZ confirm the experimental observations that it is also a DHPS substrate.


 Please wait while we load your content...
Please wait while we load your content...