Iron catalyzed diastereoselective hydrogenation of chiral imines†
Abstract
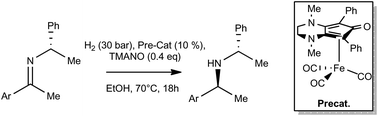
Cyclopentadienone-based iron complexes were used for the first time to successfully catalyze the diastereoselective hydrogenation of enantiopure imines. Chiral amines, including valuable biologically active products, were obtained often as enantiomerically pure compounds. Computational studies helped to elucidate the chemical and stereochemical aspects of the iron-catalyzed reaction.


 Please wait while we load your content...
Please wait while we load your content...