Tryptophan/copper-catalyzed aromatization reaction of chiral cyclohexanones to phenols†
Abstract
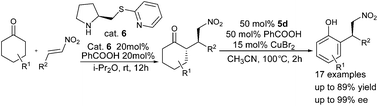
By merging organocatalysis with copper catalysis, a highly efficient stereospecific approach for the synthesis of chiral phenols from cyclohexanones has been developed for the first time. The aromatization reaction proceeds through the in situ formation of enone intermediates and further subsequent bromination/dehydrobromination reactions. And a series of functionalized phenol derivatives are obtained in good yields (up to 89%) and good to excellent enantioselectivities (up to 99% ee).


 Please wait while we load your content...
Please wait while we load your content...