Regioselective 1,2-addition of allenamides with N-haloimides: synthesis of 2-halo allylic aminal derivatives†
Abstract
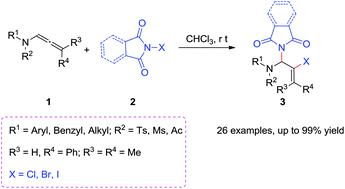
A strategy for the synthesis of 2-halo allylic aminal derivatives through regioselective 1,2-addition of allenamides with N-haloimides is presented. This reaction was conducted under very mild conditions and gave up to 99% yield. Moreover, the obtained halides allow functional group diversification by palladium-catalyzed coupling reactions, which could act as potential intermediates for the synthesis of valuable compounds.


 Please wait while we load your content...
Please wait while we load your content...