Synthesis of benzil-o-carboxylate derivatives and isocoumarins through neighboring ester-participating bromocyclizations of o-alkynylbenzoates†
Abstract
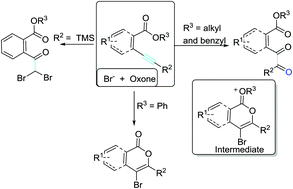
Bromide mediated neighboring ester-participating bromocyclizations of o-alkynylbenzoates are described here for the synthesis of benzil-o-carboxylates. 4-bromoisocoumarins are also synthesized when phenyl o-alkynylbenzoate is used as the substrate. Mechanistic studies suggest that the whole process is composed of an electrophilic bromocyclization and a dibromohydration-based ring-opening, and the neighboring ester group participates in the bromocyclization. Interestingly, the two oxygen atoms of the keto carbonyls in benzil-o-carboxylates are both derived from water. The electrophilic bromo source is in situ generated from the oxidation of bromide.


 Please wait while we load your content...
Please wait while we load your content...