Cobalt carbonyl-catalyzed carbonylation of functionalized aziridines to versatile β-lactam building blocks†
Abstract
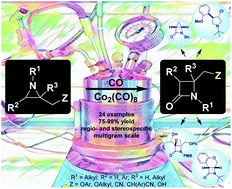
The Co2(CO)8-catalyzed carbonylation of different classes of non-activated aziridines with diverse substitution patterns was investigated. Special attention was devoted to selectivity issues and reaction optimization. This study resulted in the regio- and stereospecific synthesis of 24 novel β-lactam target structures in high yields on a multigram scale. The synthetic potential of the newly obtained azetidin-2-ones was illustrated via ring-expansion, ring-closure, and/or side chain-functionalization protocols to provide a straightforward entry to novel pyrrolidines, C-fused bi- and tricyclic β-lactams and monocyclic carbapenem analogs.


 Please wait while we load your content...
Please wait while we load your content...