A multicomponent macrocyclization strategy to natural product-like cyclic lipopeptides: synthesis and anticancer evaluation of surfactin and mycosubtilin analogues†
Abstract
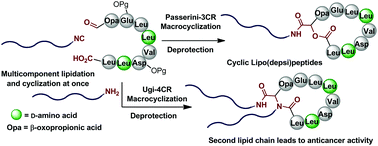
A multicomponent macrocyclization strategy towards cyclic lipopeptides is described. The approach relies on the utilization of the Ugi and Passerini multicomponent reactions for the cyclization of peptides and oxo-peptides, and here it is employed for the construction of a small library of analogues of the natural products mycosubtilin and surfactin A. A key feature of this method is the simultaneous incorporation of either one or two exocyclic lipid tails along with the macrocyclic ring closure, which is only possible due to the multicomponent nature of the macrocyclization step. The evaluation of the anticancer activity of the lipopeptide library showed that the installation of a second lipid moiety in the surfactin scaffold leads to a more potent cytotoxicity in cancer cells. This is a new example of the multicomponent reaction potential in rapidly producing natural product analogues for biological screening.


 Please wait while we load your content...
Please wait while we load your content...