Direct Csp2–H enolization: an allenoate alkylation cascade toward the assembly of multi-substituted furans†
Abstract
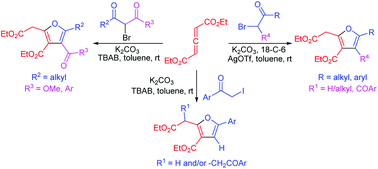
A new disconnection toward tri- and tetrasubstituted furans has been conceptualized and demonstrated. A mild, base-mediated reaction of an allenoate pronucleophile with α-halo ketones/dicarbonyl compounds results in a Csp2-alkylation/enolization/O-vinylation sequence to ultimately afford substituted furan derivatives. The reaction offers a broad scope, provides a facile access to alkyl and aryl substituted furans, and demonstrates the application of the allene functionality as a two-carbon component in the construction of furans.


 Please wait while we load your content...
Please wait while we load your content...