Domino reaction of cyclic sulfamidate imines with Morita–Baylis–Hillman acetates promoted by DABCO: a metal-free approach to functionalized nicotinic acid derivatives†
Abstract
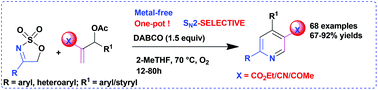
A facile, green, metal-free new one-pot synthetic strategy has been developed for easy access to a wide array of medicinally promising functionalized pyridines having an ester, a nitrile or an acetyl group at the C-3 position in good to excellent yields via a domino SN2/elimination/6π-aza-electrocyclization/aromatization reaction of several 4-aryl/hetero-aryl-substituted 5-membered cyclic sulfamidate imines with a broad range of MBH acetates of acrylate/acrylonitrile/MVK in 2-MeTHF promoted by DABCO as an organobase under an O2 atmosphere. Moreover, a biologically interesting triazolopyridine derivative was achieved through a unique procedure.


 Please wait while we load your content...
Please wait while we load your content...