Enantioselective synthesis of 1,2,3,4-tetrahydroquinoline-4-ols and 2,3-dihydroquinolin-4(1H)-ones via a sequential asymmetric hydroxylation/diastereoselective oxidation process using Rhodococcus equi ZMU-LK19†
Abstract
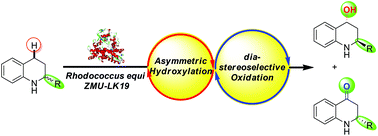
A cascade biocatalysis system involving asymmetric hydroxylation and diastereoselective oxidation was developed using Rhodococcus equi ZMU-LK19, which gave chiral 2-substituted-1,2,3,4-tetrahydroquinoline-4-ols (2) (up to 57% isolated yield, 99 : 1 dr, and >99% ee) and chiral 2-substituted-2,3-dihydroquinolin-4(1H)-ones (3) (up to 25% isolated yield, and >99% ee) from (±)-2-substituted-tetrahydroquinolines (1). In addition, a possible mechanism for this cascade biocatalysis was tentatively proposed.

 Please wait while we load your content...
Please wait while we load your content...