Mg(OMe)2 promoted allylic isomerization of γ-hydroxy-α,β-alkenoic esters to synthesize γ-ketone esters†
Abstract
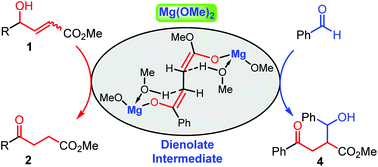
This work concerns the Mg(OMe)2 promoted allylic isomerization of γ-hydroxy-α,β-alkenoic esters with TMEDA as an additive. The isomerization proceeded under mild conditions and afforded γ-keto esters in high yield (up to 96%) within 2 h. Both (Z)- and (E)-γ-hydroxy-α,β-alkenoic esters were tolerated under the reaction conditions. This transformation involves the in situ formation of a dienolate intermediate from the easily accessible γ-hydroxy-α,β-alkenoic ester. The in situ generated dienolate can react with benzaldehyde and undergo a practical, useful tandem allylic isomerization-Aldol reaction to afford more functionalized compounds.


 Please wait while we load your content...
Please wait while we load your content...