Synthesis of perfluoroalkylated pentacenes and evaluation of their fundamental physical properties†
Abstract
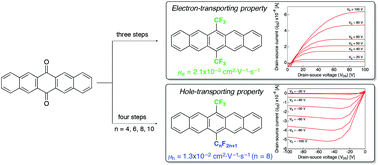
Symmetrical and unsymmetrical pentacenes carrying two perfluoroalkyl (Rf) chains, at the 6 and 13 positions, were synthesized from easily available pentacene-6,13-quinone via facile three or four step reactions. After extensive evaluation, it was clearly found that the control of both the electron density of the aromatic rings on the pentacene core and molecular alignment in the crystalline state nicely affected their physical properties. Thus, we successfully prove in this article that (1) their anti-oxidation ability was significantly enhanced due to a decrease in the HOMO and LUMO energy and (2) a distinct difference in charge-transporting properties was observed between the symmetrical and unsymmetrical pentacenes.


 Please wait while we load your content...
Please wait while we load your content...