Cobalt salt-catalyzed carbocyclization reactions of α-bromo-N-phenylacetamide derivatives†
Abstract
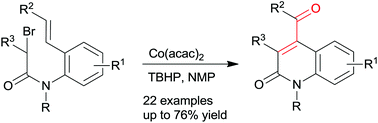
An efficient and low-cost method for the synthesis of 4-substituted quinolin-2-(1H)-ones has been developed. In the presence of TBHP, the cobalt(II)-catalyzed carbocyclization reactions of arylethenyl substituted α-bromo-N-phenylacetamides, involving sequential 6-exo-trig radical cyclization, t-butylperoxy radical cross-coupling reaction and the base-promoted ionic Kornblum–DeLaMare reaction, produced 4-benzoylquinolin-2-(1H)-ones. A variety of useful functional groups such as methoxy, fluoro, chloro, bromo, methoxycarbonyl, and cyano groups, are compatible with the reaction conditions. This strategy was further applied to arylethynyl substituted α-bromo-N-phenylacetamides, and 4-benzylquinolin-2-(1H)-ones were formed effectively.


 Please wait while we load your content...
Please wait while we load your content...