Introducing tetramethylurea as a new methylene precursor: a microwave-assisted RuCl3-catalyzed cross dehydrogenative coupling approach to bis(indolyl)methanes†
Abstract
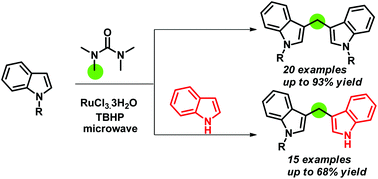
Herein we report a microwave assisted Ru(III)/TBHP-mediated reaction of indoles with tetramethylurea (TMU) synthesizing symmetrical as well as unsymmetrical bis(indolyl)methanes, where TMU acts as a methylenating agent. This is the first report where TMU is used as a methylene source. Moreover, the synthesis of unsymmetrical bis(indolyl)methanes by using a carbon precursor is also reported herein for the first time. Various substituted indoles are used for the reaction. The reaction is high yielding and takes a much shorter time to accomplish compared to the existing methods.


 Please wait while we load your content...
Please wait while we load your content...