Expedient and modular access to 2-azabicyclic architectures by iron-catalyzed dehydrative coupling of alcohol-bearing allylic lactams†‡
Abstract
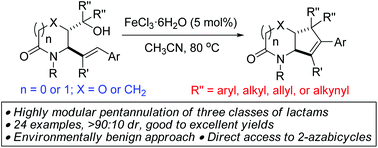
A high-yielding, cost-effective, modular and environmentally benign approach to [5,5]- or [6,5]-2-azabicyclic architectures bearing vicinal stereocenters, by Fe-catalyzed intramolecular dehydrative coupling of readily affordable alcohol-bearing allylic lactams is presented.


 Please wait while we load your content...
Please wait while we load your content...