In vivo photoacoustic tumor tomography using a quinoline-annulated porphyrin as NIR molecular contrast agent†
Abstract
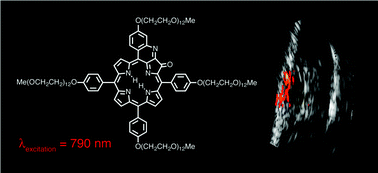
The synthesis and photophysical properties of a tetra-PEG-modified and freely water-soluble quinoline-annulated porphyrin are described. We previously demonstrated the ability of quinoline-annulated porphyrins to act as an in vitro NIR photoacoustic imaging (PAI) contrast agent. The solubility of the quinoline-annulated porphyrin derivative in serum now allowed the assessment of the efficacy of the PEGylated derivative as an in vivo NIR contrast agent for the PAI of an implanted tumor in a mouse model. A multi-fold contrast enhancement when compared to the benchmark dye ICG could be shown, a finding that could be traced to its photophysical properties (short triplet lifetimes, low fluorescence and singlet oxygen sensitization quantum yields). A NIR excitation wavelength of 790 nm could be used, fully taking advantage of the optical window of tissue. Rapid renal clearance of the dye was observed. Its straight-forward synthesis, optical properties with the possibility for further optical fine-tuning, nontoxicity, favorable elimination rates, and contrast enhancement make this a promising PAI contrast agent. The ability to conjugate the PAI chromophore with a fluorescent tag using a facile and general conjugation strategy was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...