CuBr/TBHP-mediated synthesis of N-acyl sulfonimidamides via the oxidative cross-coupling of sulfonimidamides and aldehydes†
Abstract
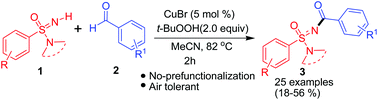
N-Acyl sulfonimidamides were synthesized via a Cu-catalyzed double C–H/N–H activation protocol. The imino end of sulfonimidamides was acylated using aldehyde as the acylating agent and t-butyl hydrogen peroxide (TBHP) as the oxidant in acetonitrile (MeCN) at 82 °C. The mild reaction conditions afforded low-to-moderate yields of N-acyl sulfonimidamides with high structural diversity.


 Please wait while we load your content...
Please wait while we load your content...