Indium-catalysed amide allylation of α-iminoamide: highly enantioselective synthesis of amide functionalised α-methylene-γ-butyrolactams†
Abstract
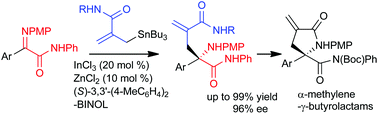
A highly enantioselective amide allylation of α-iminoamides has been achieved using catalytic amounts of InCl3, ZnCl2 and a BINOL derivative. This reaction allowed facile access to a variety of amide functionalised α-methylene-γ-butyrolactams in excellent yields with high enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...