Catalytic asymmetric aldol addition reactions of 3-fluoro-indolinone derived enolates†
Abstract
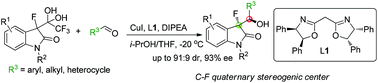
Reported herein is a Cu(I)/bisoxazoline ligand-catalyzed aldol reaction of unprotected tertiary enolates generated in situ from 3-(1,1-dihydroxy-2,2,2-trifluoroethyl)-substituted derivatives of 3-fluoro-2-oxindoles. A range of α-fluoro-β-aryl/hetaryl/alkyl-β-hydroxy-indolin-2-ones containing C–F quaternary stereogenic centers of high pharmaceutical importance were furnished in good yields and satisfactory diastereo- and enantioselectivities. The reactions were conducted under operationally convenient conditions and displayed wide substrate/functional group generality including unprotected N–H on the tertiary enolates, and aromatic, hetero-aromatic and aliphatic aldehydes.

 Please wait while we load your content...
Please wait while we load your content...