Oxidative coupling of 1-(2-methyl-4-phenylquinolin-3-yl)ethanone with ethanol and unexpected deacetylative synthesis of 3-hydroxy quinoline†
Abstract
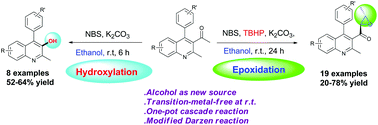
An efficient one pot method for the synthesis of anti-α,β-epoxy ketones from 1-(2-methyl-4-phenylquinolin-3-yl)ethanone and ethanol has been developed by a modified Darzen reaction. The reaction occurs under oxidative conditions via a cascade sequence of bromination, aldol condensation followed by substitution. The reaction in the presence of NBS and a base however, in the absence of an oxidant, led to the formation of the corresponding 3-hydroxylated product via an unusual rearrangement.


 Please wait while we load your content...
Please wait while we load your content...